Rules for Determining Each Quantum Number:

Two electrons can't have the same four quantum numbers because the four quantum numbers include the specification of the spin of an electron, and two electrons in the same orbital can't have the same spin. When electrons spin, they produce a magnetic field; if two positive or negative magnetic fields are next to each other, then they will repel away from each other. Think about when you try to push the positive sides of two magnets together: they try to push away from each other.


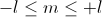
Stellar work! I especially loved the title. The only thing is that you might want to say what each quantum number is. For example, you would say that n is the principal quantum number and shows which energy level the electron is in.
ReplyDeletePaige, really nice job with the example of the two magnets to help explain how electron with the same spin repel each other.
ReplyDeleteOne thing to add to you blog and to expand on Abigail's comment, it would be helpful to describe what the four quantum numbers are such as:
n: the principle principle quantum number which describes the size of the orbital
l: the angular quantum number which describes the shape of the orbital
ml: magnetic quantum number which describes the orientation of space of an orbital of a given energy
ms:the spin quantum number which describes the spin of an electron
Also, you forgot the "l" in ml. But overall really nice job with your answer! And clever title;)